Reactivity and selectivity are two essential aspects in synthetic chemistry. It has long been explored by synthetic chemists on how to tune the reactivity and selectivity to achieve precise synthesis. As pyridine and the related N-heteroarenes are widely existing in pharmaceutical, agrochemical, and material molecule skeletons, it is of great significance for the direct functionalization of N-heteroarenes to achieve the synthesis or post-modification of important molecules. Although multiple works have been reported, it remains the ultimate challenge to achieve the high regioselectivity limited by the inherent electronic and steric properties of N-heteroarenes or specific catalyst control.
Although carbon dioxide (CO2) is a kind of greenhouse gas and brings a huge effect on the global climate, it is the ideal C1 source in synthetic chemistry due to its low cost, easy availability, nontoxicity, and recyclability. CO2 utilization is not only an essential method for serving the national strategic objective via efficient and large-scale synthesis of high-valued added bulk and fine chemicals but also has a profound and lasting effect on utilization of other renewable resources. In the field of synthetic chemistry, it is significant to use CO2 for the precise synthesis of valuable carboxylic acid. Due to the importance of hetero-aromatic carboxylic acids and the efficiency of C-H bond carboxylation, the direct carboxylation of C-H bonds in heteroarenes with CO2 attracts much interest. Although great progress has been made, new strategies is necessary to meet the requirement for the precise synthesis of related carboxylic acid based on substrate scope restriction and poor selectivity regulation in previous reports.
The group of Da-Gang Yu has been devoted to developing precise synthesis with CO2. In addition to the research of visible-light photoredox-catalyzed CO2 transformations (For work summary, see: Acc. Chem. Res. 2021, 54, 2518; For representative work, see: Nat. Catal. 2021, 4, 304;Nat. Catal. 2022, 5, 832), Yu and co-workers envisioned that organic electrochemistry, which provides electron as clean reductant, had the advantage of reactivity and selectivity control by manipulating the electric current or potential and thus carried out the research in electrochemical CO2 transformation. During the exploration (Foe representative work, see: Nat. Commun. 2021, 12, 7086; J. Am. Chem. Soc. 2022, 144, 2062), Mr. Guo-Quan Sun, who has been a Ph.D. student in Yu group since 2017, found an interesting phenomenon in 2018 that the site selectivity of N-heteroarenes carboxylation with CO2 was dictated by electrochemical reactor! Specifically, C5-carboxylated product (nicotinic acid) was obtained in divided cell and C4-carboxylated product (isonicotinic acid) in undivided cell. The Yu group later collaborated with Prof. Song Lin’s group from Cornell University and successfully developed the regiodivergent electrochemical carboxylation of N-heteroarenes and disclosed possible reasons for site selectivity via reaction condition optimizations, mechanistic studies, and theoretical calculation. In divided cell conditions, the observed carboxylation starts with the one-electron reduction of N-heteroarene, giving rise to its radical anion Int1. Density functional theory (DFT) calculations suggested that N-heteroarene radical anion Int1 bears the highest electron population at C5 position among the carbon atoms of the pyridine ring, which will thus engage in nucleophilic addition to CO2 to furnish azine radical carboxylate intermediate Int2. Subsequently, a second cathodic reduction furnishes dianion Int3, which is followed by oxidative rearomatization by O2 to provide desired carboxylation product.
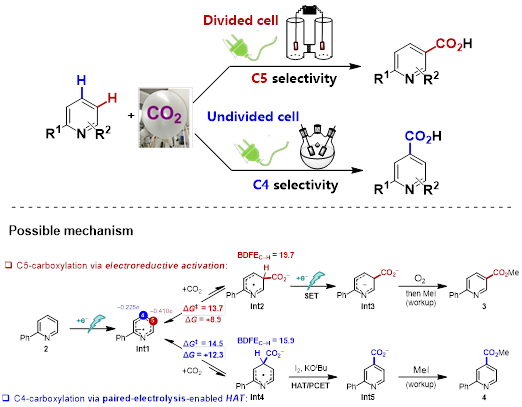
However, DFT calculations also suggested that the reaction regioselectivity could potentially be altered via the Curtin-Hammett principle. Because the nucleophilic addition of Int1 to CO2 is endergonic and reversible, the carboxylation regioselectivity could be kinetically controlled if a follow-up irreversible step favors an alternative pathway. Our DFT data revealed that if addition of CO2 occurs at the C4 position, the C–H bond at C4 of Int4 has a significantly lower bond dissociation free energy (BDFE) than the C–H bond at C5 of Int2 (ΔBDFE = 3.8 kcal/mol). Thus, if a hydrogen-atom acceptor (in undivided cell) is introduced to the reaction to promote the formation of Int5 via hydrogen-atom transfer, the reaction regioselectivity could be shifted to favor C4-carboxylation. This protocol has the advantage of mild reaction conditions, broad substrate scope, and good functional group tolerance. A series of substituted pyridines, quinolines, and other kinds of N-heteroarenes is well-compatible and transformed into the important N-heteroaromatic acids in moderate to good yields.
In summary, this work utilizes CO2 as the ideal carboxyl source to achieve regiodivergent carboxylation of N-heteroarenes by altering the electrochemical reactor. This transformation bears good substrate adaptability and functional group tolerance, which not only affords a new strategy for the synthesis of important N-heteroaromatic carboxylic acids but also provides a new train of thought in the field of CO2 resource utilization, C-H bond functionalization, and reaction selectivity control.
The research in the title of ”Electrochemical reactor dictates site selectivity in N-heteroarene carboxylations” is published in Nature (Website link: https://www.nature.com/articles/s41586-022-05667-0). Sichuan University is the first research unit. Prof. Da-Gang Yu (College of Chemistry, Sichuan University) and Prof. Song Lin (Cornell University) are the co-corresponding authors. Dr. Guo-Quan Sun (College of Chemistry, Sichuan University), Dr. Peng Yu (Cornell University), and Dr. Wen Zhang (Cornell University) are the co-first authors. We appreciate the funding from the National Natural Science Foundation of China, Sichuan Science and Technology Program, the “973” Project from the MOST of China, Fundamental Research Funds from Sichuan University and Beijing Molecular Science Center. We also thank Xiao-Yan Wang from the Analysis and Testing Center of Sichuan University, Jing Li and Dong-Yan Deng from the comprehensive training platform of the Specialized Laboratory in the College of Chemistry at Sichuan University.
